8 Sources
The report published on this website is a draft and subject to frequent updates. Please be aware that the content may change over time as revisions are made. Thank you for your understanding.
If you have questions, comments, or feedback, please contact Esteban Solorzano.
This section discusses the sources of the stakeholder needs that feed into the modeling and design of the Medical Device Systems Engineering Knowledge Repository (MDSE-KR).
8.1 Source model
Figure 8.1 is a SysML block definition diagram that shows the sources of needs and requirements for designing a MedSE knowledge repository. The blocks in the diagram represent different sources of information that can be used to identify the needs and requirements of the knowledge repository.
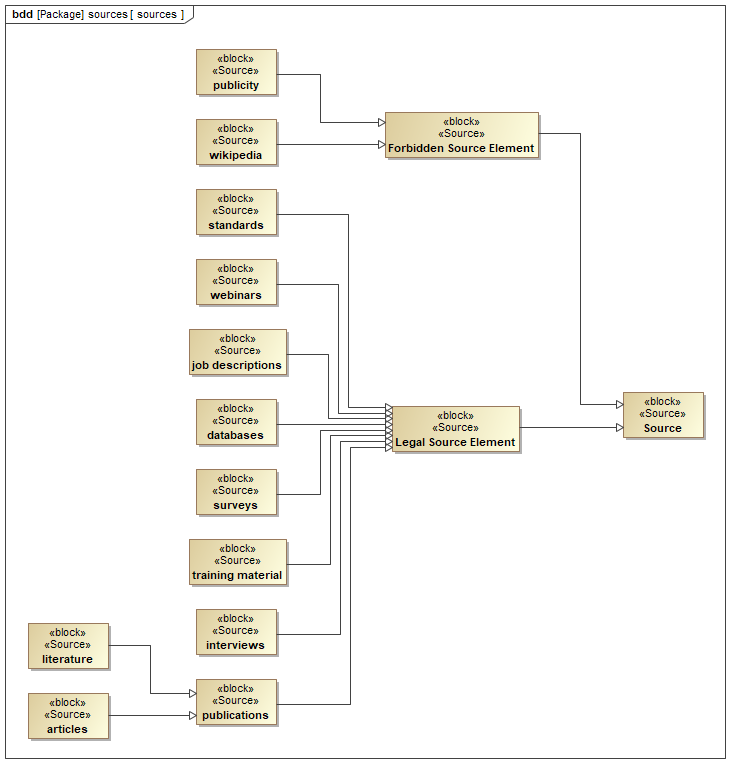
The diagram lists valid (credible) sources:
- Job descriptions
- Training material
- Literature
- Publications
- Articles
- Standards
- Databases
- Surveys
- Interviews
- Webinars
The inclusion of “Forbidden Source Element” highlights the importance of a critical evaluation when selecting information sources. Reliable and verifiable sources like publications, standards, and legal databases should be prioritized for designing the knowledge repository.
8.2 Articles
Articles that talk about the systems engineering of medical devices can serve as source for the design of the MDSE-KR. Some example articles found that can serve as source for the design of the MDSE-KR are:
Jones, D.J. and Masters, M.T. (2008), 11.1.3 Medical Device Development Process. INCOSE International Symposium, 18: 1215-1230. https://doi.org/10.1002/j.2334-5837.2008.tb00873.x (Jones and Masters 2008)
Maheshwari, Apoorv. (2015). Application of Systems Engineering to Regulatory Compliance Activities for Medical Devices. (Maheshwari 2015)
Corns, S. and Gibson, C. (2012), A Model-based Reference Architecture for Medical Device Development. INCOSE International Symposium, 22: 2066-2075. https://doi.org/10.1002/j.2334-5837.2012.tb01457.x (Corns and Gibson 2012)
“Healthcare Systems Engineering - SEBoK.” Accessed: Apr. 18, 2024. Available: https://sebokwiki.org/wiki/Healthcare_Systems_Engineering (“Healthcare Systems Engineering - SEBoK,” n.d.)
Malins, Robert & Stein, Jack & Thukral, Ajay & Waterplas, Christophe. (2015). SysML Activity Models for Applying ISO 14971 Medical Device Risk and Safety Management Across the System Lifecycle. INCOSE International Symposium. 25. 489-507. 10.1002/j.2334-5837.2015.00077.x. (Malins et al. 2015)
8.3 Medical Device Trends
The FDA provides public databases and reports that can provide insight of what are the most common medical devices that are in use and the characteristics of those devices (Health and Radiological 2024b).
8.3.1 Medical Device Classification
Figure 8.2 shows the distribution in percentage of medical devices registered with the FDA by class, as of February 1, 2024.
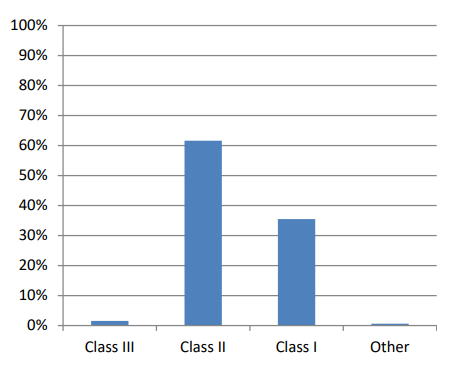
Here’s a breakdown of the data displayed in the chart:
Class III makes up the largest portion of registered devices at around 60%.
Class II is the second most common class of device at around 35%.
Class I devices and “Other” devices each make up a smaller portion of registered devices.
The FDA regulates medical devices based on the level of risk they pose to patients. Class III devices are considered the highest risk, while Class I devices are considered the lowest risk (Health and Radiological 2023).
8.3.2 Implantable versus non-implantable
Figure 8.3 is a line graph that shows the percentage of implantable and non-implantable medical devices.
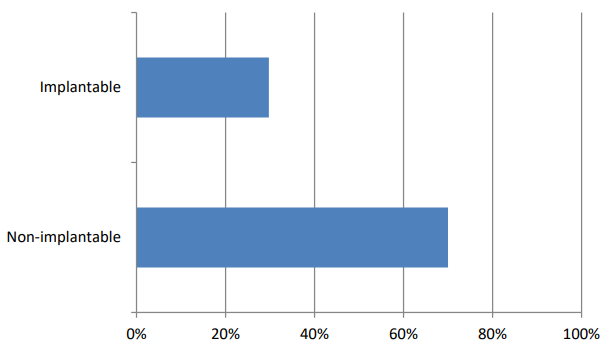
The data shows that as of February 1, 2024, implantable medical devices make up a smaller percentage of registered devices than non-implantable devices.
Implantable medical devices are devices that are inserted into the body for a long period of time. Examples of implantable medical devices include pacemakers, artificial hips, and breast implants. Non-implantable medical devices are devices that are used on the body but are not inserted into it. Examples of non-implantable medical devices include stethoscopes, blood pressure cuffs, and bandages.
8.3.3 Medical Specialties
Figure 8.4 shows the distribution of FDA-registered medical devices by medical specialty, based on FDA product codes, as of February 1, 2024.
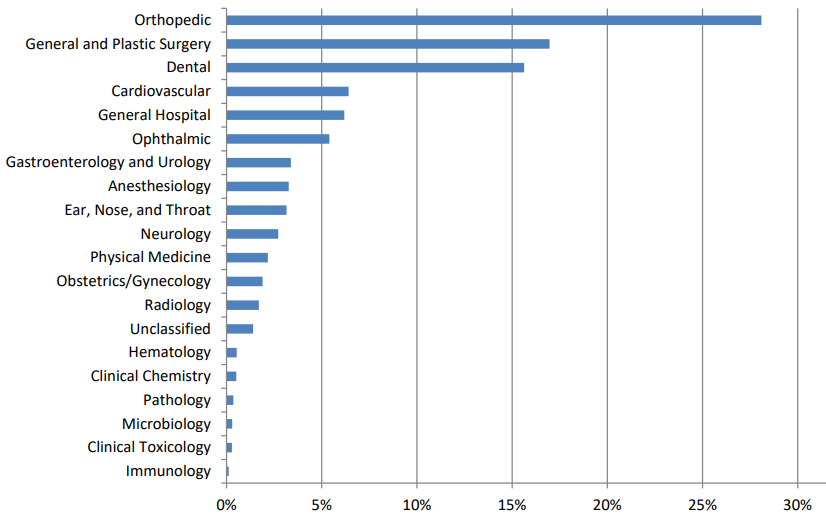
Here’s what we can gleaned from the chart:
Specialties with the most devices: The specialties with the highest percentage of registered devices are:
Orthopedic
General and Plastic Surgery
Cardiovascular
General Hospital
Specialties with the least devices: The specialties with the lowest percentage of registered devices are:
Clinical Toxicology
Immunology
Microbiology
Clinical Chemistry
Hematology
The number of devices registered in a particular specialty doesn’t necessarily reflect the number of procedures performed in that specialty. For example, there may be a relatively small number of complex orthopedic devices compared to the number of simple bandages used in wound care, but both would be classified under orthopedic devices.
Some reasons why some specialties might have more devices than others:
Surgical specialties: Surgical specialties tend to have a wider variety of devices because they use devices for a variety of procedures.
Chronic conditions: Specialties that treat chronic conditions may have a wider variety of devices because they may use devices to monitor or treat the condition over time.
8.3.4 FDA Product Code
Figure 8.5 is word cloud that highlights the most frequent terms from FDA Product Codes of registered devices.

Based on the word cloud, the most frequent FDA Product Code Terms for registered medical devices with the FDA as of February 1, 2024:
Surgical
Tracheostomy
Cardiovascular
Wireless
Synthetic
Adult
Cervical
Spinal
Electrode
Plastic
It appears that surgical devices and those used in cardiology are the most common. There is also a high prevalence of terms related to materials like synthetic and plastic, along with terms related to location or application like cervical and tracheostomy.
8.4 Webinars
Webinars that discuss systems engineering in the medical device industry can serve as sources for the needs and requirements of the MDSE-KR. Two examples of webinars that were analyzed to extract source information were the following.
8.4.1 MassMEDIC Sponsored Webinar: Systems Engineering for Medical Device Development by Sunrise Labs, Inc (“MassMEDIC Sponsored Webinar: Systems Engineering for Medical Device Development” 2022).
A webinar titled “Systems Engineering for Medical Device Development” discussed how Systems Engineering contributes to success in complex medical device development.
The webinar discussed the role of a Systems Engineer and described the Systems Engineering process. From this webinar, the MDSE-KR should incorporate the following key points:
- Foundational Concepts:
- Define Medical Device Systems Engineering and its role in the development lifecycle.
- Understand the complexities of modern medical devices (electrical, mechanical, software integration).
- Systems Engineering Process:
- Key stages involved in Systems Engineering for medical devices (concept to commercialization).
- Techniques for requirements definition, management, and traceability.
- Cross-functional Collaboration:
- Effective communication and coordination between various engineering disciplines (electrical, mechanical, software).
- Integration with other devices and user interfaces.
- Risk Management:
- Identify and mitigating risks associated with medical device development.
- Design test protocols and mitigation response plans.
- Regulatory Considerations:
- Understand and adhering to design control regulations for medical devices (e.g., ISO 13485).
- Project Management:
- Strategies for staying on budget and schedule in medical device development projects.
- Case Studies:
- Real-world examples showcasing successful application of Systems Engineering in medical device development.
8.4.2 Systems Perspective Engineering : A webinar on Medical Device product development (“Systems Perspective Engineering : A Webinar on Medical Device Product Development” 2020)
A webinar titled “Systems Perspective Engineering : A webinar on Medical Device product development” discussed how usability engineering can be integrated into the systems engineering process for medical device development.
From this webinar, the MDSE-KR should incorporate the following key points:
Usability Engineering Process:
- Iterative process applied throughout development.
- Complements design control and risk management.
- Follows IEC 62366 and FDA guidance.
- Involves user research, task analysis, risk analysis, formative evaluations, and summative validation.
Systems Engineering Approach (V Model):
- Starts with understanding user needs and defining requirements.
- Breaks down the system into manageable pieces.
- Focuses on mitigating technical risks early.
- Integrates different disciplines throughout development.
Why Integrate Usability into Systems Engineering? * Usability is a project-level risk that should be mitigated early. * Usability engineering tools complement the V model. * Early integration reduces design risk and rework later.
Who Owns Usability?
- Ideally, the systems engineer due to their focus on mitigating risks.
- Can be someone else with influence to plan and integrate usability activities.
Usability Engineering Strategy:
- Should be planned early and scaled based on device risk.
- Defines the types of evaluations and resources needed throughout development.
- Integrates usability activities throughout all development phases.
Recommendations for Reducing Usability Risk:
- Consider usability risk mitigation early in system architecture planning.
- Shift usability evaluation of critical hardware subsystems earlier.
- Evaluate software user interfaces before coding and implementation.
- Develop instructions for use and training as subsystems to be iterated on.
- Strategically sequence development of features based on usability risk.
- Plan ahead for materials and control of devices for usability studies.
Additionally, the webinar covered:
- Examples of usability engineering tools used throughout the development process.
- How to develop a usability engineering plan.
- How to conduct usability studies at different development phases.
8.5 Courses directed to systems engineers who work in the medical device industry
A company called Technology and Management Training Courses and Seminars (TONEX) provides a course called *“Systems Engineering for Medical Device Development” (“Systems Engineering for Medical Device Development,” n.d.). The 2-day course is designed to equip professionals in the medical device industry with the essential knowledge and skills in systems engineering.
The course has the following learning objectives:
Understand the fundamentals of systems engineering as applied to medical device development.
Navigate regulatory requirements and standards specific to the medical device industry.
Implement risk management strategies to enhance the safety and efficacy of medical devices.
Apply systems thinking to optimize the integration of diverse components in the development process.
Design and execute comprehensive verification and validation protocols for medical devices.
Enhance communication and collaboration among cross-functional teams involved in medical device development.
This course is for professionals and decision-makers involved in various stages of medical device development, including:
Biomedical Engineers
Regulatory Affairs Specialists
Product Managers
Quality Assurance Professionals
Systems Engineers
Project Managers
Course Outline:
Introduction to Systems Engineering in Medical Devices
Overview of Systems Engineering Principles
Importance of Systems Thinking in Medical Device Development
Key Challenges and Opportunities in the Field
Regulatory Landscape for Medical Devices
Overview of FDA and International Regulatory Agencies
Current Good Manufacturing Practices (cGMP)
Ensuring Compliance with ISO Standards
Risk Management in Medical Device Development
Identifying and Assessing Risks in the Development Process
Risk Mitigation Strategies and Implementation
Integration of Risk Management with Regulatory Requirements
Systems Integration and Interdisciplinary Collaboration
Interconnected Components in Medical Device Systems
Strategies for Effective Cross-Functional Collaboration
Role of Systems Engineering in Integration Challenges
Verification and Validation in Medical Device Development
Designing Comprehensive Verification Protocols
Conducting Rigorous Validation Testing
Ensuring Compliance with Regulatory Requirements
Communication and Documentation in the Development Lifecycle
Effective Communication Strategies for Cross-Functional Teams
Documentation Best Practices and Regulatory Requirements
Creating a Culture of Transparency and Accountability in Development Teams
An analysis of the course objectives and outline derived the following MDSE-KR needs and requirements.
Needs:
Gain a deep understanding of how systems engineering principles are applied to the specific context of medical device development.
Master the Medical Device Development Life Cycle (MDLC) with a focus on the role of systems engineering at each stage.
Effectively navigate the regulatory landscape governing medical devices (e.g., FDA QSR (Health and Radiological 2024a), ISO 13485).
Implement robust risk management strategies to ensure the safety and efficacy of medical devices.
Foster successful integration of diverse components within a complex medical device system.
Enhance communication and collaboration skills to work effectively with cross-functional teams.
Stay updated on best practices for verification and validation (V&V) of medical devices.
Understand how systems engineering needs to adapt to emerging trends in medical device technology.
Requirements:
Content:
In-depth coverage of foundational systems engineering principles with a focus on medical device applications.
Detailed breakdown of the MDLC with clear explanations of the systems engineering role at each stage.
Dedicated chapters on risk management, including relevant techniques and regulatory considerations.
Comprehensive discussion of systems integration challenges and best practices in a medical device context.
Practical guidance on V&V processes for medical devices, including different testing types and regulatory compliance.
Emphasis on communication and documentation best practices for effective cross-functional collaboration.
Inclusion of case studies showcasing successful medical device development projects with strong systems engineering involvement.
A dedicated chapter on emerging trends in medical device technology and their implications for systems engineering.
Appendix with relevant regulatory resources (FDA regulations, ISO standards).
Format:
Organized structure with clear headings, subheadings, and bullet points for easy navigation.
Use of diagrams, figures, and tables to illustrate concepts.
Glossary of key terms specific to systems engineering and medical device development.
Index for information retrieval.
Availability in both print and digital formats.
Usability:
Written at a level appropriate for experienced systems engineers, but with clear explanations for core concepts.
Real-world examples and case studies to enhance understanding and practical application.
Chapter summaries and review questions to solidify learning.
Links to online resources (e.g., regulatory websites, industry best practices documents) for further exploration (optional for digital format).
8.6 Companies that offer systems engineering services for medical device development
8.7 Job Profiles of Systems Engineers of medical device companies
Appendix Medical Device Systems Engineer (MDSE) Job Descriptions contains a sample of eight job descriptions that showcase the variety of roles and responsibilities under the title “Systems Engineer” in the medical device industry. The source of the Job Descriptions was from LinkedIn.
Here’s a breakdown of key findings of the eight job descriptions:
Commonalities:
All positions require a strong understanding of engineering principles and practices.
Most roles emphasize experience working on complex systems, often involving hardware, software, and electrical components.
Excellent communication and collaboration skills are crucial for success in this field, as systems engineers frequently interact with various teams (e.g., design, manufacturing, regulatory).
Ability to manage projects, meet deadlines, and prioritize effectively is essential.
Key Differences:
Experience Level: The roles range from requiring 6+ years of experience (Job Description #4) to 10+ years (Job Descriptions #3, #5, #8).
Focus Area: Responsibilities can be specific to a certain medical device type (e.g., cardiac mapping - Job Description #1, heart failure management - Job Description #6) or broader, encompassing various technologies (Job Description #7).
Project Stage: Some positions focus on the entire development lifecycle (Job Description #5), while others target specific stages like early concept development (Job Description #7) or system verification and validation (Job Description #6).
Leadership: Some roles involve leading and mentoring engineers (Job Descriptions #3, #5, #7, #8), while others focus on individual technical contributions (Job Descriptions #1, #4, #6).
Additional Insights:
Several positions highlight the importance of adhering to medical device regulations like FDA and ISO standards (Job Descriptions #3, #5, #6, #8).
Some roles emphasize experience with specific technologies relevant to medical devices, such as Bluetooth or implantable sensors (Job Descriptions #6, #7).
The eight job descriptions for Medical Device Systems Engineers were examined to identify the required qualifications, responsibilities, and skills. The information is presented in several tables for clarity.
8.7.1 MDSE Responsibilities
Table 8.1 ranks from most common to least common the responsibilities based on how often they appeared in the job descriptions. Participating in cross-functional teams, managing projects, and developing system requirements are the most common. Less frequent responsibilities include conducting training and managing intellectual property.
| Responsibility | # of Job Descriptions |
|---|---|
| Participate in cross-functional teams | 8 |
| Manage projects | 7 |
| Develop system requirements | 7 |
| Communicate effectively with various teams | 7 |
| Lead design reviews | 6 |
| Perform risk analysis | 6 |
| System verification and validation | 6 |
| Develop design documentation | 5 |
| Troubleshoot and solve technical problems | 5 |
| Translate user needs into technical specifications | 4 |
| Manage external vendors | 3 |
| Create and maintain technical documentation | 3 |
| Conduct training | 2 |
| Partner with research and development teams | 2 |
| Identify unmet user needs | 1 |
| Lead brainstorming sessions | 1 |
| Manage intellectual property | 1 |
8.7.2 MDSE Systems Engineering Skills
Table 8.2 focuses on skills specific to systems engineering, ranked from most common to least common. Complex systems thinking, system requirements development, and system design and architecture are the most common. Test plan development and execution, and trade-off analysis are less frequently mentioned.
| Skill | # of Job Descriptions |
|---|---|
| Complex systems thinking | 7 |
| System requirements development | 7 |
| System design and architecture | 6 |
| Risk analysis and management | 6 |
| System verification and validation | 6 |
| Interface management (between subsystems) | 3 |
| Systems modeling and simulation | 2 |
| Test plan development and execution | 2 |
| Trade-off analysis (considering various factors) | 1 |
| Systems integration and deployment | 1 |
8.7.3 MDSE Medical Device Skills
Table 8.3 highlights medical device specific skills, ranked from most common to least common. General medical device experience and knowledge of regulatory affairs (FDA, ISO) are common requirements. Experience with specific technologies or domains are less frequent.
| Skill | # of Job Descriptions |
|---|---|
| Medical device experience (general) | 4 |
| Regulatory affairs knowledge (e.g., FDA, ISO standards) | 4 |
| Experience with specific medical device technologies (e.g., implantable sensors) | 2 |
| Experience in a specific medical device domain (e.g., Renal Care) | 1 |
8.7.4 MDSE Soft Skills
Table 8.4 categorizes soft skills required for the role from most common to least common, with communication, collaboration, and problem-solving being the most sought-after. Public speaking and teaching/mentoring are the least common skills mentioned.
| Skill | # of Job Descriptions |
|---|---|
| Communication (written and oral) | 8 |
| Collaboration | 8 |
| Problem-solving | 7 |
| Analytical skills | 6 |
| Attention to detail | 5 |
| Technical expertise (engineering principles) | 5 |
| Project management | 6 |
| Leadership | 4 |
| Regulatory knowledge (FDA, ISO) | 4 |
| Creativity | 2 |
| Public speaking | 1 |
| Teaching/Mentoring | 1 |
8.7.5 MDSE Qualifications
Table 8.5 ranks the qualifications listed in the job descriptions by frequency. A Bachelor’s degree in Engineering and strong communication skills are the most common requirements, while an advanced degree (Ph.D. or Post-Graduate) and experience in a specific medical device domain are the least common.
| Qualification | # of Job Descriptions |
|---|---|
| Bachelor’s degree in Engineering or related field | 8 |
| Strong communication skills | 8 |
| Ability to work effectively in a team | 7 |
| Experience with complex systems | 7 |
| Project management skills | 6 |
| Master’s degree in Engineering or related field (or equivalent experience) | 4 |
| Medical device experience | 4 |
| Regulatory affairs knowledge (e.g., FDA, ISO standards) | 4 |
| Experience with specific medical device technologies (e.g., implantable sensors) | 2 |
| Leadership experience | 2 |
| Advanced degree (Ph.D. or Post-Graduate) | 1 |
| Experience in a specific medical device domain (e.g., Renal Care) | 1 |
8.7.6 MDSE Preferred Qualifications
Table 8.6 summarizes qualifications that are desirable but not always mandatory from most common to least common. A Master’s degree, leadership experience, and expertise in specific medical device technologies are preferred by some employers.
| Qualification | # of Job Descriptions |
|---|---|
| Master’s degree in Engineering or related field (or equivalent experience) | 4 |
| Leadership experience | 2 |
| Experience with specific medical device technologies (e.g., implantable sensors) | 2 |
| Advanced degree (Ph.D. or Post-Graduate) | 1 |
| Experience in a specific medical device domain (e.g., Renal Care) | 1 |
| Proficiency in generating system/subsystem specifications | 1 |
| Familiarity with advanced needs and requirements definition methods | 1 |
The following sources for the MDSE-KR was obtained from the analysis of the eight job descriptions:
Foundational Engineering Knowledge:
Solid grounding in engineering fundamentals: This includes mechanics, electronics, thermodynamics, and materials science.
In-depth coverage of a specific engineering discipline: Mechanical, electrical, or biomedical engineering would be most relevant.
Systems Engineering Skills:
Systems engineering mindset: Understandi complex systems, their interactions, and life cycle.
System requirements development: Capture user needs, translate them into technical specifications, and perform requirements analysis.
System design and architecture: Design and modeling the overall system and its subsystems.
Risk analysis and management: Identify potential risks throughout the development lifecycle and implement mitigation strategies.
Systems verification and validation: Ensure the system meets requirements and performs as intended.
Project management: Planning, scheduling, budgeting, and managing resources for successful project completion.
Medical Device Specific Knowledge:
Medical device regulatory landscape: Focus on key regulations like FDA 510(k) and ISO 13485.
Common medical device technologies: Sensors, actuators, microprocessors, communication protocols.
Biocompatibility principles: Selection of materials that are safe for human interaction within the body.
Ethical considerations in medical device design: Ensuring patient safety, privacy, and data security.
Emerging trends in medical device technology: Artificial intelligence, machine learning, and telehealth.
Career paths for medical device systems engineers: Explore various specializations and leadership opportunities.
Case studies of successful medical device development projects.
Real-world examples and exercises to reinforce learning and practical application.
Guidance on professional development resources and certifications relevant to the field.
8.8 Survey
This section dives into the insights gleaned from a survey conducted in April 2024. The survey targeted systems engineers working in the field of medical device development. The survey received responses from a diverse group of professionals from leading companies including Boston Scientific, the FDA, Nudge BG, BIOTRONIK, and others. The primary objective of this survey was to identify the knowledge and resources that are most valuable to medical device systems engineers in their daily work.
The analysis of the survey responses was aimed to inform the development of the MDSE-KR.
The following section will present a detailed analysis of the survey findings.
For reference, the template used for the survey questions is included in Appendix Survey of this report. This will allow readers to gain a deeper understanding of the specific questions asked and the context surrounding the survey responses.
