6 Problem
The report published on this website is a draft and subject to frequent updates. Please be aware that the content may change over time as revisions are made. Thank you for your understanding.
If you have questions, comments, or feedback, please contact Esteban Solorzano.
6.1 Problem Statement
In recent years, the field of medical device systems engineering has gained significant attention due to the increasing complexity of medical devices and the rigorous regulatory requirements they must meet. As a result, there has been a noticeable growth in the number of books and resources dedicated to medical devices . Topics covered in these books may include regulatory compliance, risk management, quality assurance, and design principles tailored to medical devices. But there is very few books on systems engineering for medical devices.
Systems engineering is a broad discipline applied across various industries, including aerospace, automotive, defense, telecommunications, and more. Therefore, there are numerous books available on systems engineering principles, methods, and applications in these fields.
The number of books on systems engineering of medical devices is fewer in comparison to those covering systems engineering in more established industries.
6.1.1 Problem from the perspective of non-systems engineers
Lack of a clear understanding of what is systems engineering of medical devices:
- Colleagues and senior leadership aren’t clear…
- If it is ‘requirements’ (or even models), why does that add value?
- Medical Device Systems Engineering can look like more paperwork (or more modeling work)
- And have a separate function? Why not hire more software and hardware people “who actually deliver things the customer uses”?
6.1.2 Problem from the perspective of systems engineers
Medical Device Systems Engineers don’t seem to agree what it is…but everyone seems sure they know and are right.
6.2 Problem modeled as a casual loop
Figure 6.1 is a diagram of a causal loop (Sterman and Sterman 2000) that shows a system where investment in systems engineering yields positive results. The diagram shows that increased profits can lead to more resources for better systems engineering which help reduce unsuccessful medical devices.
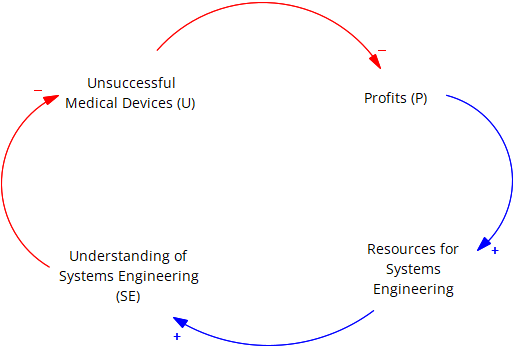
Analysis of the Causal Loop in the Image:
An explanation of the elements that make up the casual loop is given as follows:
Unsuccessful Medical Devices (U) –> Decreased Profits (P) (Negative) - Unsuccessful devices lead to lower sales and profitability.
Profits (P) –> Resources for Systems Engineering (SE) (Positive) - More profits allow for more investment in systems engineering.
Understanding of Systems Engineering (SE) –> Decreased Unsuccessful Medical Devices (U) (Negative) - Better understanding of systems engineering leads to fewer poorly designed devices. If there’s a lack of understanding of systems engineering principles among stakeholders in the medical device industry, it can lead to ineffective utilization or implementation of systems engineering methodologies in the development process.
Resources for Systems Engineering (SE) –> Improved Understanding of Systems Engineering (SE) (Positive) - More resources dedicated to systems engineering education improves understanding.
Feedback Loops:
- Balancing Loop (B): This loop discourages unsuccessful devices. As unsuccessful devices (U) increase, profits (P) decrease. This decrease in profits (P) leads to fewer resources for systems engineering (SE). Less resources for systems engineering (SE) leads to a poorer understanding of systems engineering (SE), which ultimately leads to more unsuccessful devices (U). This loop creates pressure to improve systems engineering practices to break the cycle of unsuccessful devices.
Key Points:
The diagram shows a reinforcing cycle (B) that discourages unsuccessful devices. This is because better understanding of systems engineering leads to fewer unsuccessful devices, which increases profits, which allows for more investment in systems engineering, and so on.
Low adoption of systems engineering results in ineffective medical device development processes. Without proper systems engineering practices, the development processes may lack holistic consideration of all system components, leading to inefficiencies, errors, and delays.
Ineffective development processes can contribute to increased incidents of device failures. Without robust systems engineering practices, there’s a higher likelihood of design flaws, inadequate testing, and safety issues in medical devices, leading to higher failure rates and potential harm to patients.
6.3 Contributing factors
6.3.1 Systems engineering is used more in other fields
Systems engineering is employed more in other fields than the medical device industry. Some of the top fields where systems engineering is used include:
Aerospace and Defense: Systems engineering plays a crucial role in designing and developing complex aircraft, spacecraft, missiles, and defense systems.
Automotive Industry: In the automotive sector, systems engineering is essential for designing vehicles with integrated and optimized systems for safety, performance, and efficiency.
Information Technology: Systems engineering is utilized in IT for designing, implementing, and managing large-scale computer systems, networks, and software applications.
Telecommunications: Systems engineering is vital for designing and optimizing telecommunications networks, including mobile networks, satellite systems, and internet infrastructure.
Energy Sector: Systems engineering is employed in the energy industry for designing and managing complex energy production and distribution systems, including power plants, renewable energy systems, and smart grids.
Transportation: Systems engineering is crucial in designing and optimizing transportation systems, including railways, highways, airports, and public transportation systems.
Manufacturing: Systems engineering is used in manufacturing for designing and optimizing production processes, supply chain management systems, and industrial automation systems.
Environmental Engineering: In environmental engineering, systems engineering is applied to design and manage complex environmental systems, such as water treatment plants, waste management systems, and pollution control systems.
Robotics and Automation: Systems engineering is essential for designing and integrating robotic systems and automation solutions across various industries, including manufacturing, healthcare, and agriculture.
6.3.2 Very few papers on systems engineering of medical devices
There are very few papers on the application of systems engineering to medical devices that are published as articles, and/or presented in conferences.
In the 34th Annual INCOSE International Symposium, of the 83 papers presented none were in the context of medical devices. Of the 48 presentations, only 2 were in the context of medical devices (is2024?-).
An article database query was performed using a software called IHS Goldfire (“Goldfire Cognitive Search Accuris,” n.d.) to determine how many peer reviewed articles (including articles within books) discuss systems engineering of a medical device. IHS Goldfire is a software platform developed by IHS Markit that contains tools for knowledge discovery, problem-solving, technology scouting, innovation management, and intellectual property management. Table 6.1 shows the query results per application field. Between 1980 and 2024 there were a total of 3432 articles that discuss systems engineering of a medical device. This is significantly less than the 40732 peer reviewed articles found for systems engineering for military applications.
| Application Field | Articles in books | Articles and Journals | Total |
|---|---|---|---|
| Medical Devices | 2799 | 633 | 3432 |
| Military | 14908 | 25824 | 40732 |
| Aerospace | 12443 | 19254 | 31697 |
| Automotive | 13082 | 9433 | 22515 |
| Software | 24587 | 48953 | 73540 |
| Telecomunications | 15897 | 10841 | 26738 |
| Transportation | 20139 | 23044 | 43183 |
| Manufacturing | 21205 | 28341 | 49546 |
6.3.3 Lack of books and literature of systems engineering of medical devices
The are very few books that discuss or teach systems engineering in the context of medical device development. Of the few books found are the following:
“Robust Systems Engineering for Medical Device Design” by Mr. Martin A. Coe. Description: This book introduces practical systems engineering methods for the design and development of commercially engineered systems, focused on the design of medical devices. It begins with systems engineering definitions, fundamentals and proceeds by integrating systems engineering activities into the development process to demonstrate a successful system design (Coe 2019).
“Healthcare Systems Engineering” by Paul M. Griffin, et al. Description: Offers comprehensive coverage of the healthcare system, healthcare delivery, and healthcare systems modeling (“Healthcare Systems Engineering | Wiley,” n.d.).
The INCOSE Systems Engineering Handbook only has one page and a half that talks about Biomedical and Healthcare Systems (INCOSE Systems Engineering Handbook 2023).
